FAQs
Frequently Asked
Questions
Get answers to some of the most commonly asked questions about PAH, TYVASO DPI, and TYVASO.


STARTING TYVASO
What is prostacyclin-class therapy?
People living with PAH may not produce enough natural prostacyclin. TYVASO is a man-made form of prostacyclin, called a prostacyclin mimetic. It mimics some of the effects of the natural prostacyclin in the body, helping to keep the blood vessels in the lungs open and working properly.
What can I expect with TYVASO therapy?*
Adding TYVASO to your background oral medication, like bosentan (an ERA) or sildenafil (a PDE-5 inhibitor), could help improve your ability to exercise. Adding TYVASO may help you do more in your day—go for a stroll, walk your kids to the bus stop, or walk to a mailbox.
In a clinical study, people who added TYVASO to their PAH background therapy walked farther—improving their 6MWD† by 22 m at 12 weeks, with improvements seen in as soon as 6 weeks.‡
LEARN MORE ABOUT TYVASO RESULTS
Individual results may vary.
6-minute walk distance (6MWD) is a test to see how far you can walk in 6 minutes.
In the 12-week clinical study of TYVASO, people taking an oral PAH medicine (bosentan or sildenafil) completed a 6-minute walk test at the beginning and end of the study.
How do I get TYVASO?
If you and your doctor agree that TYVASO could help you, a referral for your TYVASO prescription will go to a Specialty Pharmacy (SP) provider or United Therapeutics Cares™. Once approved, an SP nurse will come to your home or your doctor’s office to show you how to take TYVASO and will support you as you begin therapy.
What should I tell my doctor before starting TYVASO?
Before you take TYVASO or TYVASO DPI, tell your healthcare provider about all of your medical conditions, including if you:
- Have low blood pressure
- Have or have had bleeding problems
- Have asthma or chronic obstructive pulmonary disease (COPD)
- Are pregnant or plan to become pregnant. It is not known if either product will harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if either product passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment.
See Important Safety Information and Full Prescribing Information for TYVASO and TYVASO DPI for more information.
Where do I get my TYVASO device?
TYVASO DPI and the TYVASO nebulizer are available only through the following Specialty Pharmacy providers:


TYVASO DPI
What is TYVASO DPI?
TYVASO DPI features the same medicine as the TYVASO nebulizer but in a dry powder inhaler. This small, portable inhaler uses cartridges and features a simple-to-use design.
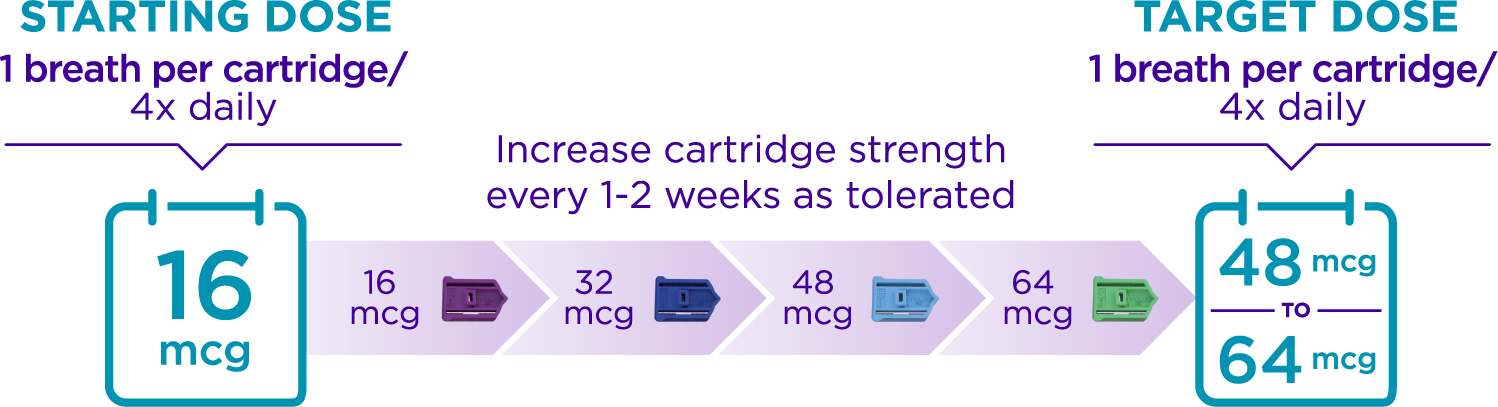
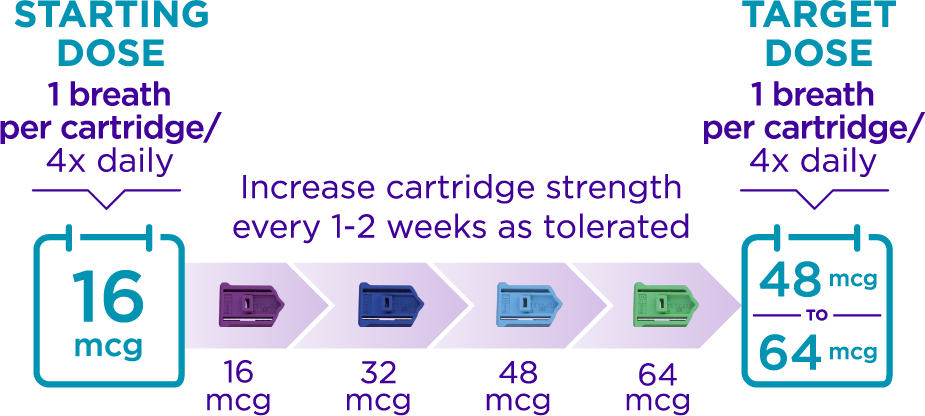
How does TYVASO DPI dosing work?
Your doctor will work with you to increase your dose until you reach your target maintenance dose. This process is called “titration.”
Plan to take your medication approximately every 4 waking hours.
- Doses are taken with 1 breath per cartridge
- Most people start with the 16 mcg cartridge, 4x daily, and increase their dose every 1-2 weeks
How do I switch from the TYVASO nebulizer to TYVASO DPI?
Talk to your doctor to learn about switching to TYVASO DPI. In most cases, your doctor will prescribe the cartridge strength that equals the number of breaths you are currently taking with your TYVASO nebulizer.
How do I use TYVASO DPI?
TYVASO DPI uses cartridges and features a simple-to-use design. Taking a dose is as simple as just one breath—open, load, inhale.
For more information, download the TYVASO DPI Instructions for Use.
How do I store TYVASO DPI?
Blister cards and strips:
Store at room temperature. When at room temperature, sealed and unopened blister cards and strips must be used within 8 weeks, and opened strips must be used within 3 days.
Additional blister cards and strips may be stored in the refrigerator until the expiration date printed on the blisters, but should be room temperature for 10 minutes before use.
Do not put a blister card or strip back into the refrigerator after being opened or stored at room temperature.
Inhaler:
The TYVASO DPI inhaler may be stored at room temperature or in the refrigerator. If refrigerated, the inhaler should be left at room temperature for 10 minutes before use.
The inhaler can be used for up to 7 days from the date of first use. After 7 days of use, the inhaler must be discarded and replaced with a new inhaler.
TYVASO Nebulizer
How does the TYVASO nebulizer fit into my daily routine?
You can take the TYVASO nebulizer with you throughout your day. The portable TYVASO device allows for dosing at home or on the go.
- Set up device once a day
- Each treatment session takes about 2-3 minutes
- Plan for treatment sessions approximately every 4 waking hours
- Clean device once a night
How do I store TYVASO ampules?
TYVASO comes in plastic ampules packaged in a light-protective foil pouch and are good until the date shown, when stored in the unopened pouch at the temperature range indicated on the pouch. TYVASO ampules do not need to be refrigerated, and should be stored away from light.
TYVASO ampules can be used until the expiration date printed on the pouch. Do not use TYVASO ampules past the expiration date printed on the pouch.
- TYVASO ampules should be used within 7 days after opening the foil pouch
- TYVASO can be kept in the TYVASO nebulizer medicine cup for no more than 1 day
Throw away any remaining TYVASO that is left in the medicine cup at the end of the day.
The Instructions for Use manual will tell you all you need to know to properly assemble, use, care for, and troubleshoot your TYVASO nebulizer. You can also watch this instructional video for more information.
How do I care for my TYVASO nebulizer?
The Instructions for Use manual will tell you all you need to know to properly assemble, use, care for, and troubleshoot your TYVASO nebulizer. Watch this instructional video or download the Instructions for Use manual.
Key things to remember:
- At the end of each day, you’ll need to go through a short process of taking apart the unit and washing the various accessories. Once you get used to this, it usually takes about 5 minutes
- Once a week, use a lint-free cloth to wipe out the inside of the inhalation device chamber
- Once a month, you’ll get a set of new accessories, along with your TYVASO Refill Kit
Side Effects
What are the possible side effects of TYVASO or TYVASO DPI?
Both products can cause serious side effects, including:
- Low blood pressure (symptomatic hypotension). If you have low blood pressure, either product may lower your blood pressure more.
- Bleeding problems. Either product may increase the risk of bleeding, especially in people who take blood thinners (anticoagulants).
The most common side effects of both products are cough, headache, throat irritation and pain, nausea, reddening of the face and neck (flushing), fainting or loss of consciousness, dizziness, diarrhea, and shortness of breath. Like other inhaled prostaglandins, you may have trouble breathing after taking TYVASO or TYVASO DPI because it may cause the muscles around your airway to tighten (bronchospasm). These are not all the possible side effects. Call your doctor for medical advice about side effects or if you have trouble breathing.
Tell your doctor about any side effect that bothers you or does not go away.
See Important Safety Information and Full Prescribing Information for TYVASO and TYVASO DPI for more information.
Is there anything I should tell my doctor about the other medications I take?
TYVASO was added to treatment with other PAH medications in multiple studies.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Either product and other medicines may affect each other.
Especially tell your healthcare provider if you take:
- Medicines used to treat high blood pressure or heart disease
- Medicines that decrease blood clotting (anticoagulants)
- Water pills (diuretics)
- Gemfibrozil (Lopid®) or rifampin (Rimactane®, Rifadin®, Rifamate®, Rifater®)
Be sure to make a list of all the medicines (prescription and over the counter), vitamins, and herbal supplements you take routinely or even occasionally. Update the list as needed, keep it with you, and show it to your doctor and Specialty Pharmacy provider.
See Important Safety Information and Full Prescribing Information for TYVASO and TYVASO DPI for more information.
Access and Patient Support
Will someone teach me how to use TYVASO?
Once your doctor has prescribed TYVASO, your medicine will be delivered to your home, and a Specialty Pharmacy (SP) nurse or United Therapeutics Cares Patient Navigator will contact you to schedule a virtual or in-person appointment to teach you how to take it. You can receive additional visits and phone calls to be sure you are comfortable using TYVASO, and you can call for help 24/7.
Where can I get more info about TYVASO?
Watch videos and download helpful resources on the TYVASO RESOURCES page.
Your healthcare team and doctor are always available to answer questions about your TYVASO treatment.
How do I refill my TYVASO prescription?
Each month, your Specialty Pharmacy (SP) provider will automatically send you a 28-day supply of TYVASO, along with a kit containing a new set of accessories or inhalers for your TYVASO treatment. Don’t hesitate to contact your SP provider if you have any questions about taking TYVASO, operating the devices, or if you need more medication.
What if I have to replace my TYVASO device?
If you’re using the TYVASO nebulizer, your Specialty Pharmacy (SP) provider will replace your device automatically every 2 years, and you will receive a refill kit once each month, which includes a new set of accessories.
If you’re using TYVASO DPI, you will receive a refill kit each month that includes 5 inhalers, which will allow you to use a new inhaler every week, with a spare inhaler just in case.
TYVASO DPI and the TYVASO nebulizer are available only through the following SP providers:


You are now leaving the Tyvaso.com website
This link will take you to a site maintained by a third party who is solely responsible for the site’s content. Click CONTINUE to proceed or CLOSE to return to www.TYVASO.com.
You are now leaving the Tyvaso.com website
This link will take you to a site maintained by a third party who is solely responsible for the site’s content. Click CONTINUE to proceed or CLOSE to return to www.TYVASO.com.